Glaucoma Stem Cell Clinical Trials

Stem cell ophthalmology treatment study.
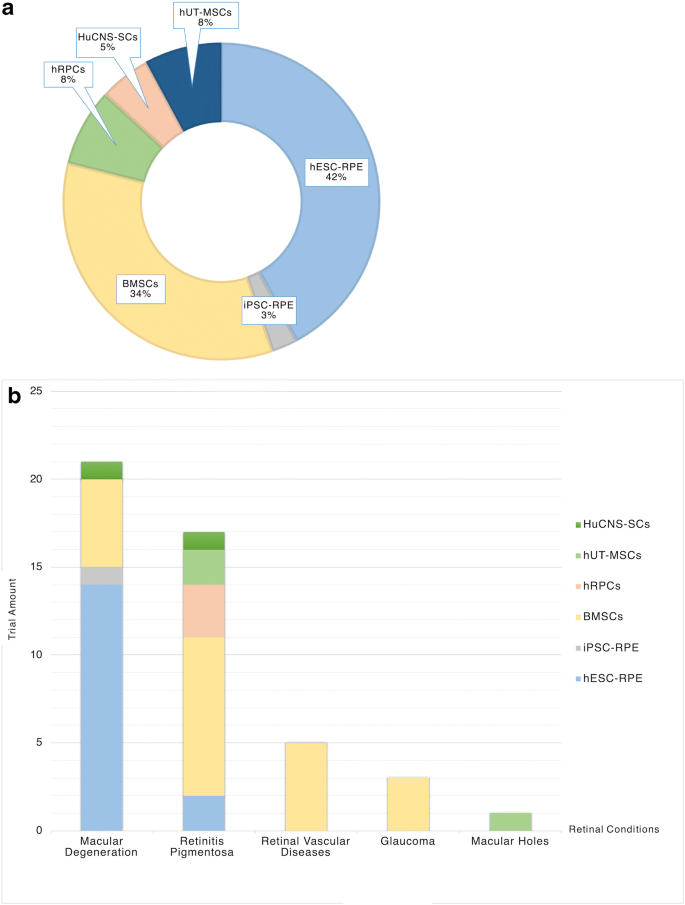
Glaucoma stem cell clinical trials. A world first clinical trial led by the center for eye research australia has shown that vitamin b3 nicotinamide could play an important role in protecting against nerve cell damage that leads. Glaucoma can currently be managed with treatments including eye drop medication laser and surgery and early treatment can prevent vision loss in most cases. Stem cell ophthalmology treatment study scots. Human stem cells have shown promise and deserve attention not just in the laboratory but in the clinical setting as well.
We are delighted with the results of this study which offers real hope to millions whose sight has been damaged by glaucoma. This article provides an overview of stem cells for the treatment of glaucoma via neuroprotection neuroenhancement and possibly cell replacement strategies. Indeed for some clinical trials patients are reimbursed for costs associated with study visits such as parking or travel. Patients with glaucoma should only consider receiving stem cell treatment in the context of a well regulated clinical trial after consultation with their personal ophthalmologist.
Clinical trials and you nih has this resource if you are interested in finding a clinical trial for your medical condition. This research also has the potential to improve the success of eye transplants helping a transplanted eye connect to the brain by growing axons through the optic nerve. The international society for stem cell research isscr isscr has developed information to help you evaluate claims you may have seen regarding stem cell treatments. Patient funded trials are studies in which patients pay to participate.
Hope for the future. Layout table for additonal information. If you are considering a stem cell treatment for glaucoma please think about the following very important factors. Recently we heard from a glaucoma patient who enrolled in a patient funded trial in which the person paid 20 000 and received stem cell injections around one eye.
Fda warns about stem cell claims fda has this resource describing the regulation of stem cell. Bone marrow derived stem cells in the treatment of leber s hereditary optic neuropathy. In a typical clinical trial patients are not asked to pay for the treatment. This research now needs to be taken to clinical trial to enable it to become an accessible safe and approved treatment for patients.